Natural Steric Analysis (STERIC)
-
Reference
J. K. Badenhoop and F. Weinhold, J. Chem. Phys. 107, 5406-5421, 5422-5432 (1997);
J. K. Badenhoop and F. Weinhold, Int. J. Quantum Chem. 72, 269-280 (1999).
-
Notes
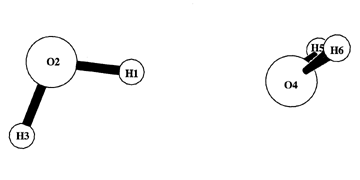
STERIC analysis presents two tables describing localized NBO/NLMO contributions to "steric exchange energy," the energy change associated with N-electron exchange antisymmetry of the wavefunction (Pauli exclusion principle). In accordance with Weisskopf's picture [Science 187, 605-612 (1975)], steric exchange is evaluated in terms of "kinetic energy pressure," the additional oscillatory and nodal features needed to preserve mutual orthogonality as electronic orbitals crowd into a spatial region. The first table of STERIC output evaluates steric exchange energy in terms of full NLMO (vs. pre-orthogonal PNLMO) contributions, incorporating all aspects of N-electron interatomic orthogonality. The second table presents a pairwise-additive estimate (based on partial orthogonality involving only two pairs simultaneously) that is less accurate, but more closely related to the concept of steric "contact" between individual electron pairs. These two steric-type energies refer to different asymptotic "zero" reference points, and only their differences (e.g., between crowded and uncrowded geometries) can be directly compared. The Sample Output illustrates STERIC output for a model water dimer (RHF/6-311++G**) in the geometry shown below. (See NBO 6.0 Manual, pp. B100-103 and the references for additional details.)
-
Sample Input (G9X)
%chk=h2o_h2o
#N rhf/6-311++G** POP=NBORead
H2O...HOH
0 1
H 0.036726 0.556520 0.000000
O -0.006183 1.525447 0.000000
H 0.909465 1.817564 0.000000
O -0.006183 -1.376675 0.000000
H -0.423629 -1.782131 0.767110
H -0.423629 -1.782131 -0.767110
$NBO steric $END