General Multi-Center NBO Search (NCBOND)
-
Reference
E. D. Glendening and F. Weinhold, J. Comp. Chem. ??, ???? (2010?).
-
Notes
The NCBOND (n-center bond) keyword automates the search for exotic n-center bonds (n ≤ 9) that transcend the 2-center bonds of ordinary covalent molecules. A multi-center natural Lewis structure (NLS) is considered superior to a standard 2-center NLS representation if, and only if, it leads to (1) appreciable reduction in overall NL-occupancy, and (2) an increase (or at least no decrease) in the number of above-threshold L-type NBOs, and (3) a decrease (or at least no increase) in the number of above-threshold NL-type NBOs. If no such improved NLS is found in the successive search from n = 3 to n = 9, the program returns with the best ordinary 2-center NLS, as found in the default NBO search.Known prototypes for multi-center bonding include the famous 3-center bridge-bonds of diborane (B2H6) and other borohydrides. The search for 3-center, 2-electron (3c/2e) bonds formerly required the 3CBOND keyword, and such a search could not be extended beyond n = 3. In NBO 6, however, the search proceeds sequentially through n = 3, 4, 5,...,9, terminating at intermediate n only if a clearly superior NLS is found.
Other familiar multi-center bonds are found in the 6-center aromatic rings of benzenoid systems and the 3c/4e "hyperbonds" of many transition metal species. Unlike the 3-center bonds of diborane, however, these latter cases can be well described by two dominant NRT resonance structures, e.g., the two Kekule structures of benzene. All cases of n-center bonding may be said to exhibit extraordinary delocalized character, but many cannot be reduced (as for benzene) to a few NRT structures of conventional 2-center form. In this respect, the 3-center bonds of diborane are more representative of the surprisingly unconventional n-center bonding motifs that NCBOND search may uncover for n > 3.
NCBOND usage is illustrated below for the 5-center bonds of B6? "wagon-wheel" species (B3LYP/3-21G level). See the NBO 6.0 Manual, pp. B???? for additional discussion of NCBOND keyword usage.
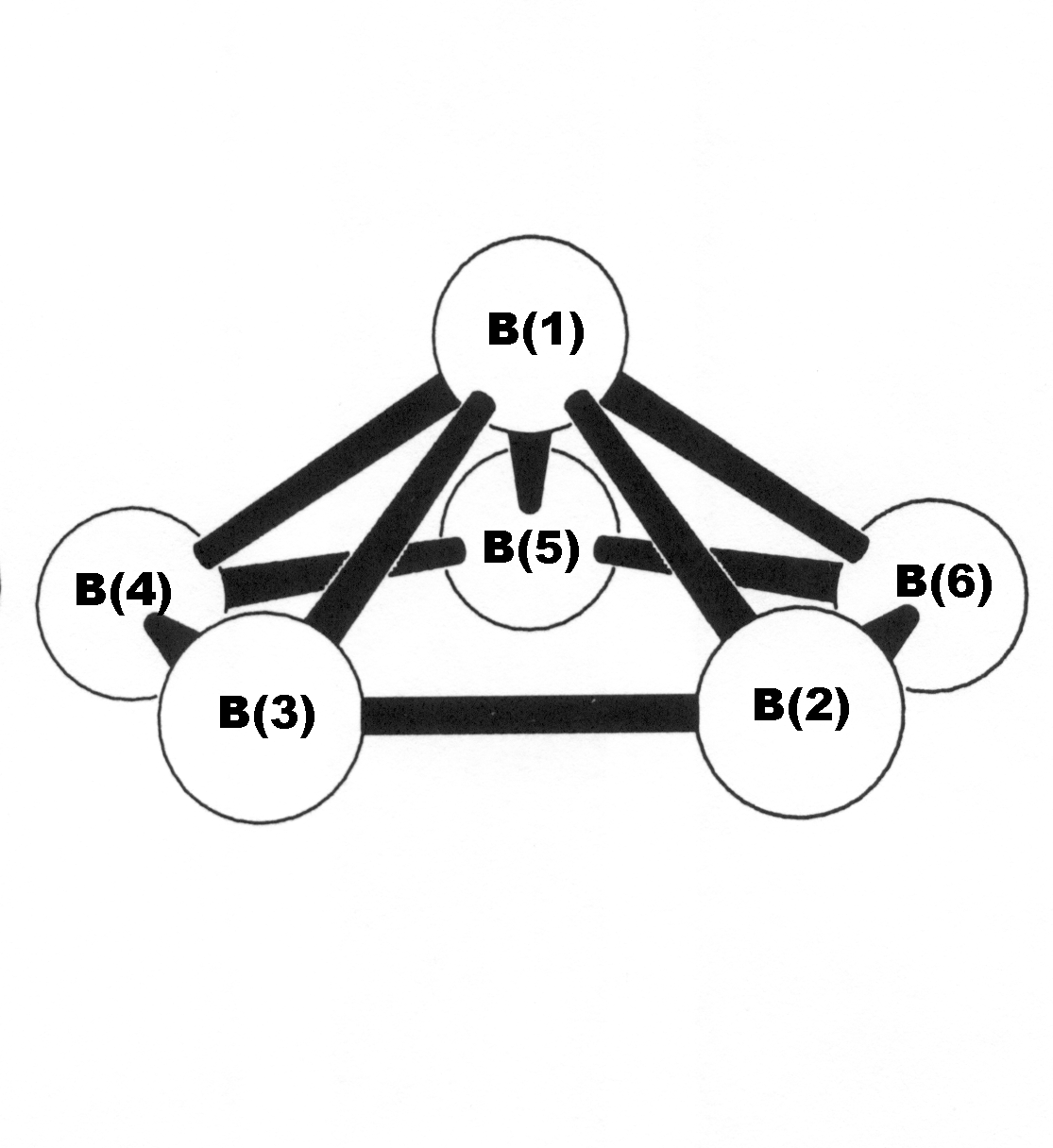
-
Sample Gaussian Input
#b3lyp/3-21g pop=nboread
B6 (C5v) "wagon wheel"
0 1
B 0.000000 0.000000 0.780348
B 0.000000 1.369175 -0.156070
B 1.302163 0.423098 -0.156070
B 0.804781 -1.107686 -0.156070
B -0.804781 -1.107686 -0.156070
B -1.302163 0.423098 -0.156070
$nbo ncbond $end